# Title: 03102019- III pBK549 transformation on ylic133 for further sanity check in SATAY 😊 👊
# Date
03102019-09102019
# Objective
To ensure that the constructed strain is able to pass the Satay sanity check, and then I can continue with the further steps, like mating with yEK7a.
# Method
- @14:00 Incubation form glycerol stocks of ylic133_1, ylic133_4,ylic133_5 and Byk832 in new YPD+6xADE media.
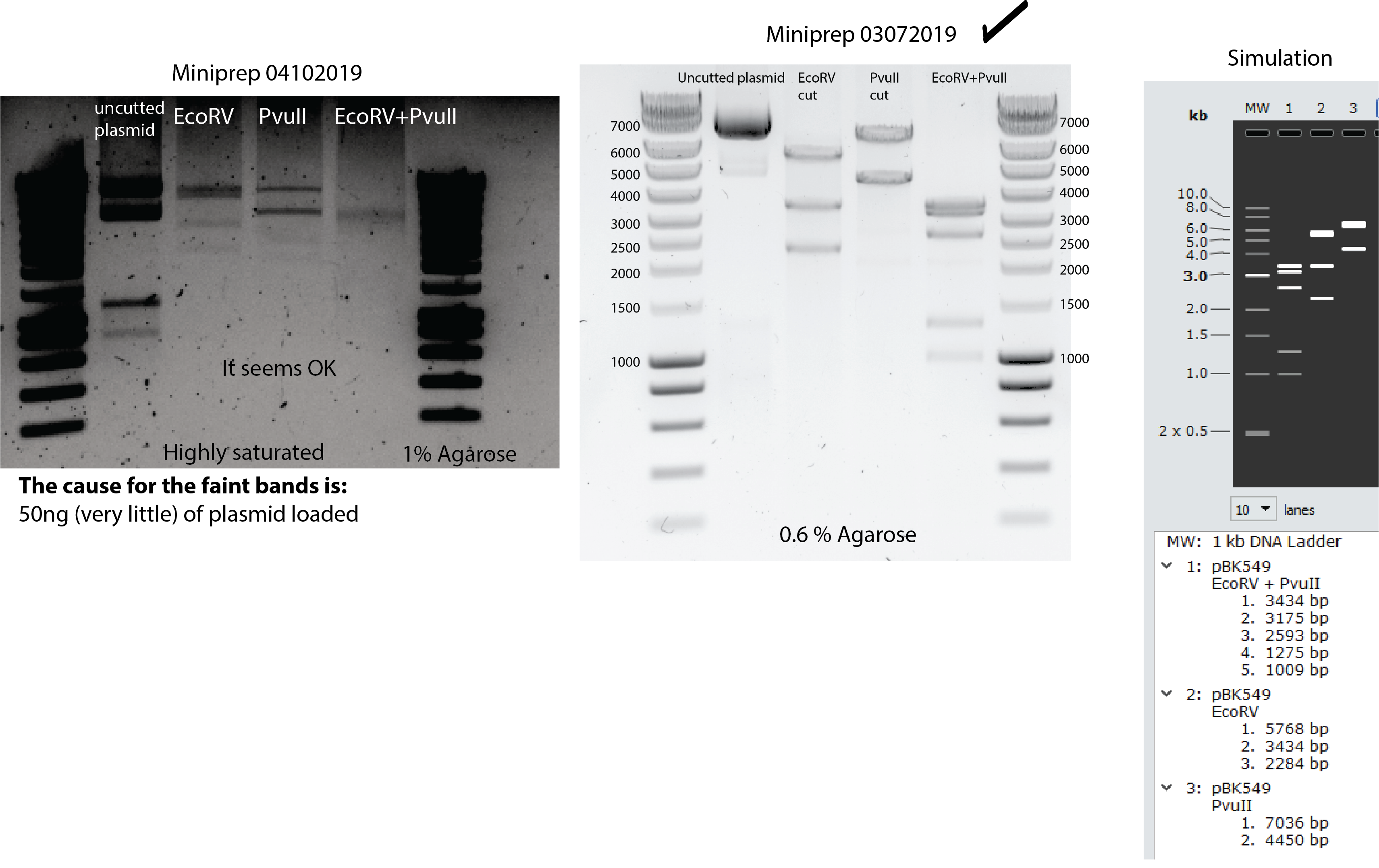
- New pBK549 plasmid extraction from bacteria. Miniprep and enzyme restriction assay in the same day of transformation. Look here for the restriction protocol
- 04102019 @9:15 OD measurements
| OD-10X dilution | Titer | Dilution factor to OD=0.5 | Time | |
|---|---|---|---|---|
| ylic133_1 | 0.368 | 3.68 | 7.76X | @9:15 |
| ylic133_5 | 0.478 | 4.78 | 9.56X | @9:15 |
| Byk832 | 0.057 | 0.57 | 1,14X | @9:15 |
- Miniprep to extract pBK549
- concentration: 46,7 ng/ul total volume 240 uL
- Digestion testing by EcoRv, PvuII and both:
- @13:00 OD measurements 10x dilution
| OD-10X dilution | Titer | Ready to transform | Time | |
|---|---|---|---|---|
| ylic133_1 | 0.255 | 2.5 | Yes | @9:15 |
| ylic133_5 | 0.198 | 1.98 | Yes | @9:15 |
| Byk832 | 0.385 | 3.8 | Yes | @9:15 |
- 3ul plasmid (46,7ng/ul) implies 140ng plasmid.
- I prepare another stock of 1M of LiAc.
- Plating 150ul cells+50ul MiliQ and 30ul cells+170ul MiliQ in -URA+6xADE and 20ul cells +180ul MiliQ in YPD (positive control)
# Results 😄
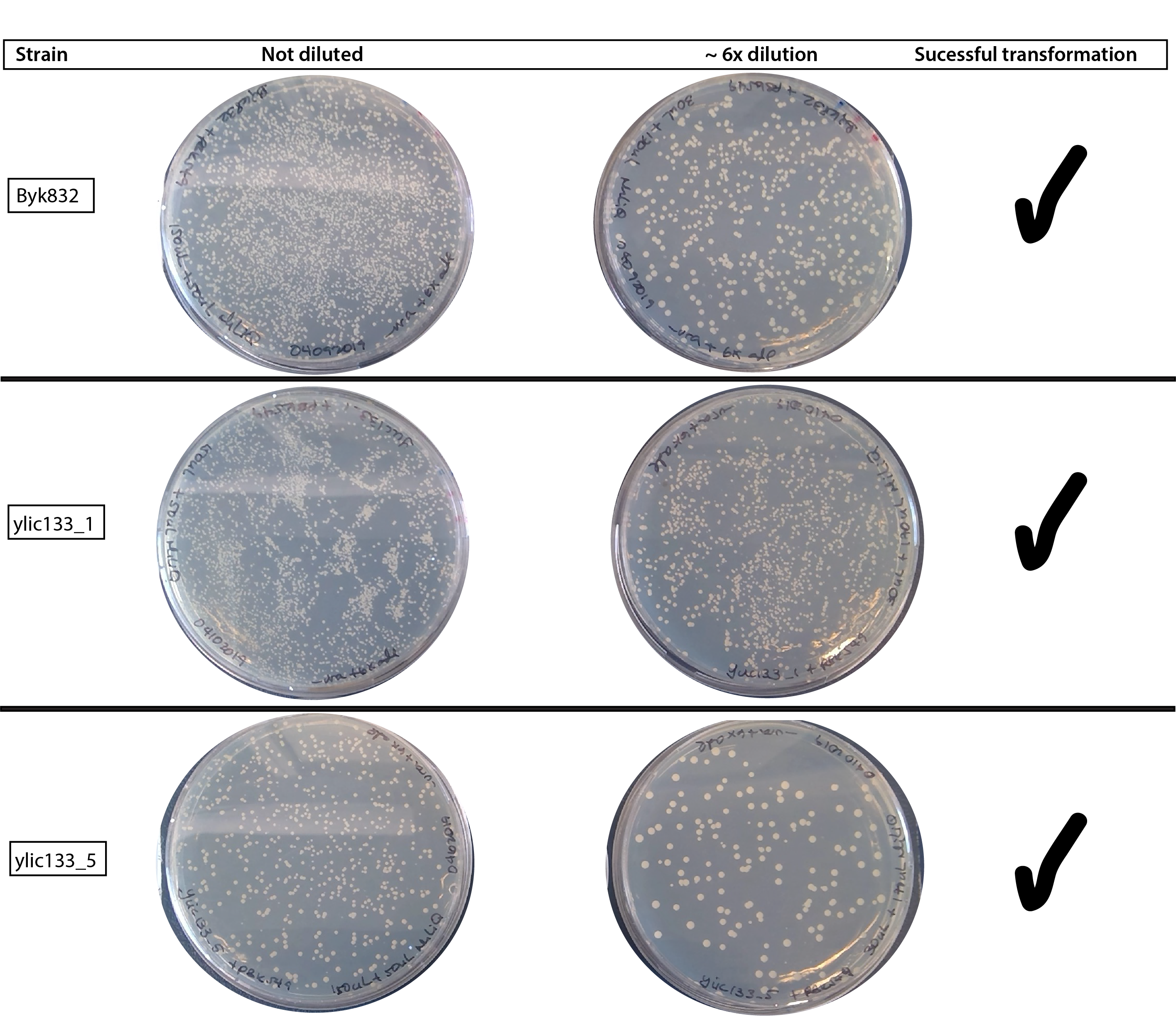
# Conclusion
- 09102019 This time the transformation really works , giving plenty of colonies in all the strains. I suspected that what made the big difference is the change of the LiAc.
- The negative control is good, so there is no growth in any of the plates.
# Next steps
- Sanity check in -ade and -ura plates .