# Title : 01102019- II pBK549 transformation on ylic133 for further sanity check in SATAY 😔
# Date
01102019-03102019
# Objective
To ensure that the constructed strain is able to pass the Satay sanity check, and then I can continue with the further steps, like mating with yEK7a.
# Method
- 30092019 -Incubation of single colonies from the plates from 24092019 and Byk832 as positive control from glycerol stock @10:30.
- 01102019 - OD measurements @9:35
| OD-10X dilution | Titer | Dilution factor to OD=0.5 | Time | |
|---|---|---|---|---|
| ylic133_1 | 1.214 | 12.14 | 24 | @9:35 |
| ylic133_4 | 1.216 | 12.16 | 24 | @9:35 |
| ylic133_5 | 0.316 | 3.16 | 6 | @9:35 |
| Byk832 | 0.308 | 3.08 | 6 | @9:35 |
- @9:55 Incubation at OD~= 0.5 to OD~=2 in YP+6x ADE
| OD-10X dilution | Titer | Ready to transform | Time | |
|---|---|---|---|---|
| ylic133_1 | 0.121 | 1.21 | close to OD=2 | @13:15 |
| ylic133_4 | 0.118 | 1.18 | close to OD=2 | @13:15 |
| ylic133_5 | 0.233 | 2.33 | yes | @13:15 |
| Byk832 | 0.290 | 2.9 | yes | @13:15 |
- @14:30 30C incubation
- 100ng of pBk549
- Notes on the procedure :
- I did not vortex the cells with the transformation mix, instead I pippeted in and out after each ingredients, by this way I avoided clumps formation that hinder transformation efficiency.
- I did not use the maximum speed (13300) of the centrifuge to get rid of the LiAc, instead I used 8000rpm.
- I heated the ssDNA to 95C and then transfered to ice before I added the 0.1M LiAc , so I could do all steps of LiAc washing and samples separation consecutively.
- I plated all (200ul) in the selection plates (-URA+6x ADE), both, negative control and transformed cells.
# Results
- 031022019 No clonies in the selection plates 😔 I just see 3 colonies in ylic133_1 plate.
Possible causes:
- The plasmid is degraded
- check: I loaded 10ul of plasmid into a gel to see If I have low bands, that could indicate degradation of the plasmid.
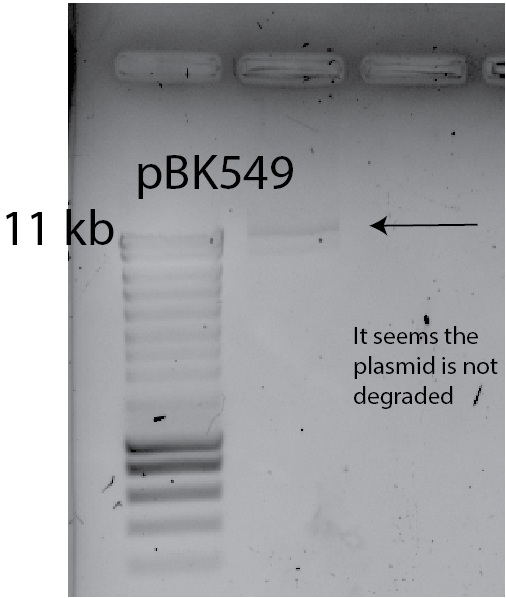
- This result implies that plasmid degradation IS NOT THE CAUSE.
- check: I loaded 10ul of plasmid into a gel to see If I have low bands, that could indicate degradation of the plasmid.
- The plasmid was lost
- check: I restreak one colony I had on the selection plate into a new -URA+6xade plate to see if they actually acquired the plasmid:
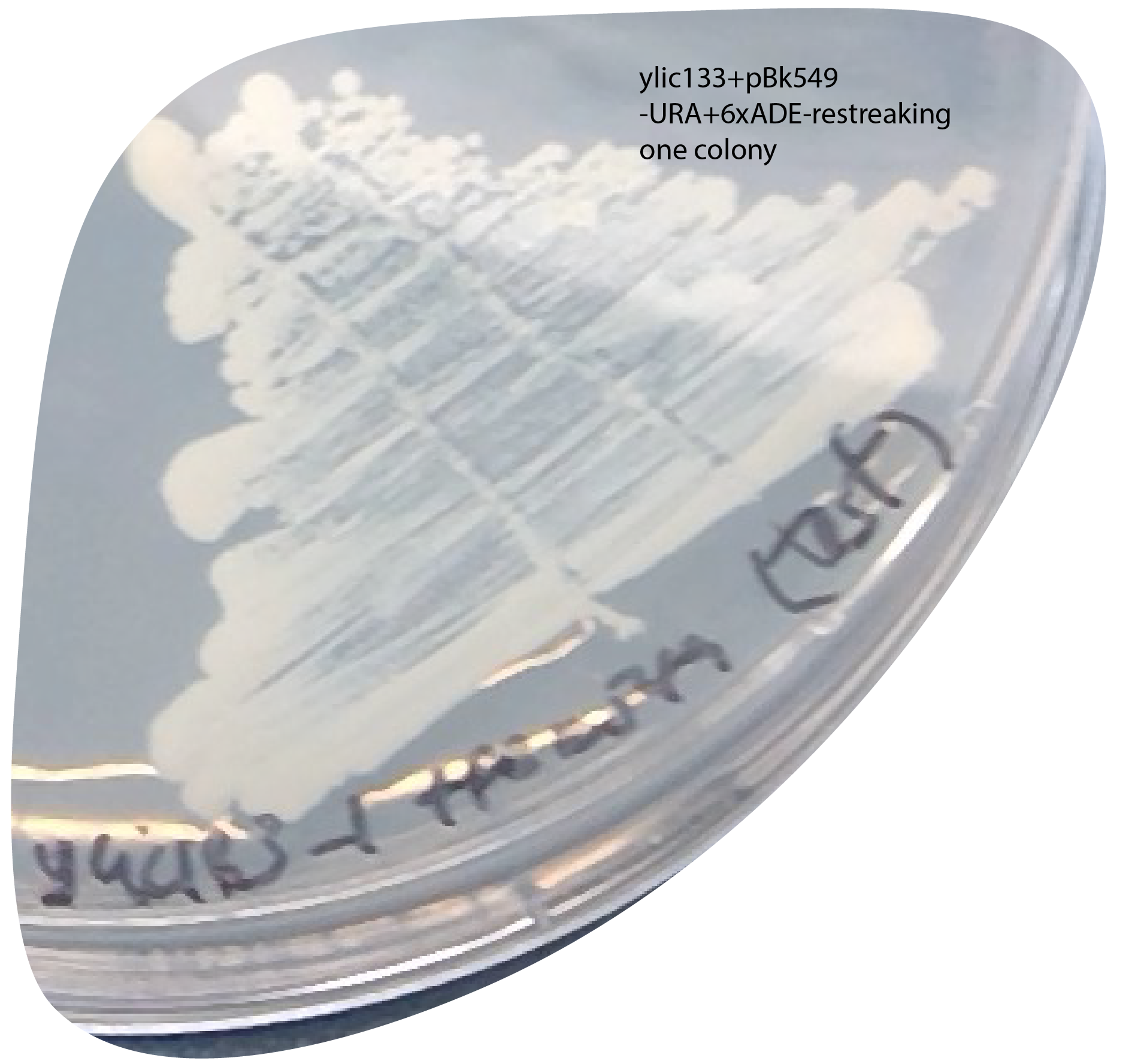
- This implies that is neither the cause.
- check: I restreak one colony I had on the selection plate into a new -URA+6xade plate to see if they actually acquired the plasmid:
- I used an extremely low amount of plasmid
- This could be due that I did not vortex the plasmid before using it, and maybe some of it sediment to the bottom, and effectively I took less plasmid than what I thought. Next time I should vortex it anyways.
- The selection plates are not good i.e. nothing can grow there.
- check: I plated a positive control (yWKD017 and ylic132) on the -ura plate+6x Ade I used for the selection plate.
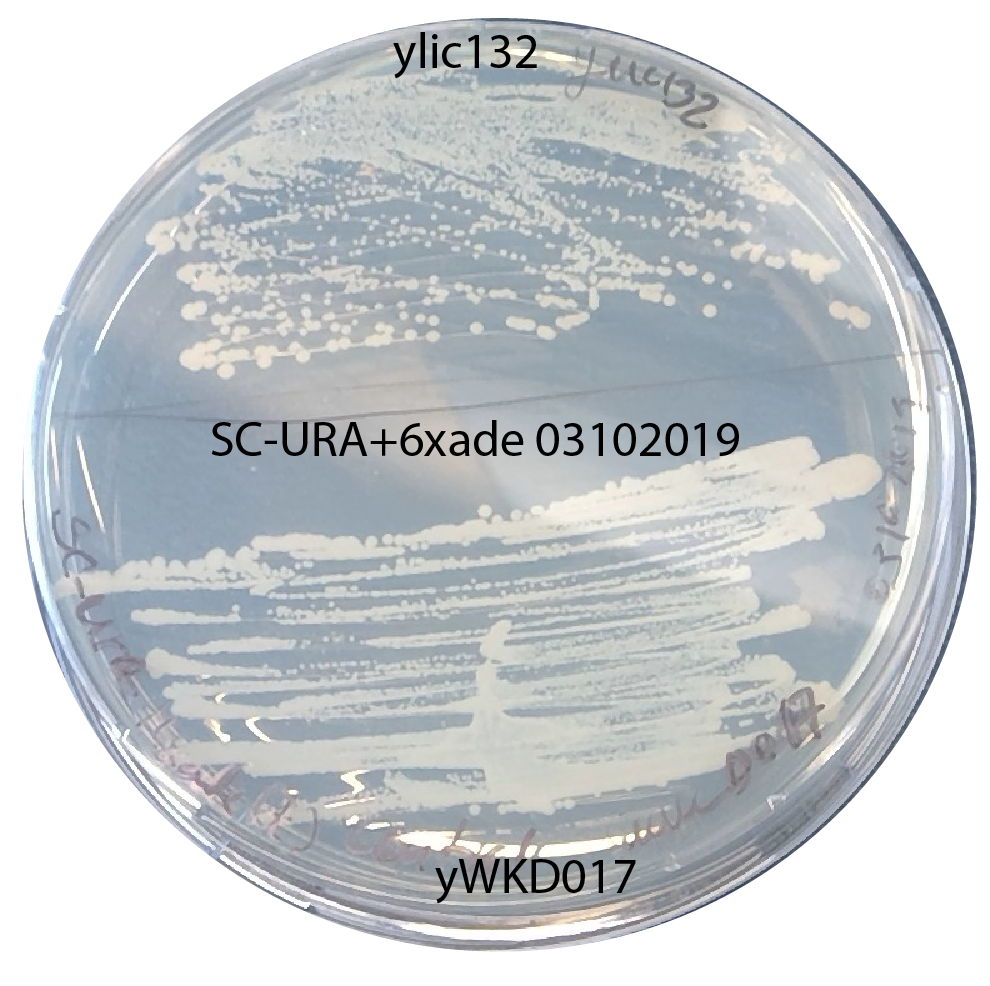
- This growth of positive control on the plate indicates that the plates ARE NOT THE CAUSE
- check: I plated a positive control (yWKD017 and ylic132) on the -ura plate+6x Ade I used for the selection plate.
- My reagents of the transformation protocol are not good.
- Maybe my PEG50% is not well... If the next transformation does not work , I will replace all my reagents for new fresh ones.
- I actually replace the LiAc , I did a new 1M stock of 50mL.
- The plasmid is degraded
# Conclusion
- Still I dont know the cause of why my transformation arent working, though I discarded multiple causes.
- I will try again with new plasmid from another bacteria incubation, and after checking that my plates are indeed correct, to discard those factors.