Title : 12092019-Transformation of ylic133 with the pLL112 plasmid :pensive:
Contents
68. Title : 12092019-Transformation of ylic133 with the pLL112 plasmid :pensive:#
68.1. Date#
12092019-20092019
68.2. Objective#
To mark temporarily ylic133 in order to be able to select for diploids after the mating with yEK7a.
68.3. Method#
Plasmid transformation using pLL112: pRS416 (URA3 CEN6_ARSH4)
12092019: Incubation of ylic133_2 in 10mL YP+2% Dextrose
13092019: OD measurements 9:30am
OD-10X dilution
Titer
Dilution factor to OD=0.5
Time
ylic133_2
0.259
2.6
5.2
9:30
2h after the 5x dilution of the dense culture in 10mL YPD
OD-10X dilution
Titer
Time
ylic133_2
0.126
1.3
11:30
OD-10X dilution
Titer
Time
ylic133_2
0.239
2.4
13:30
5ul of pLL112 plasmid , which has 2290ng/mL
68.4. Results#
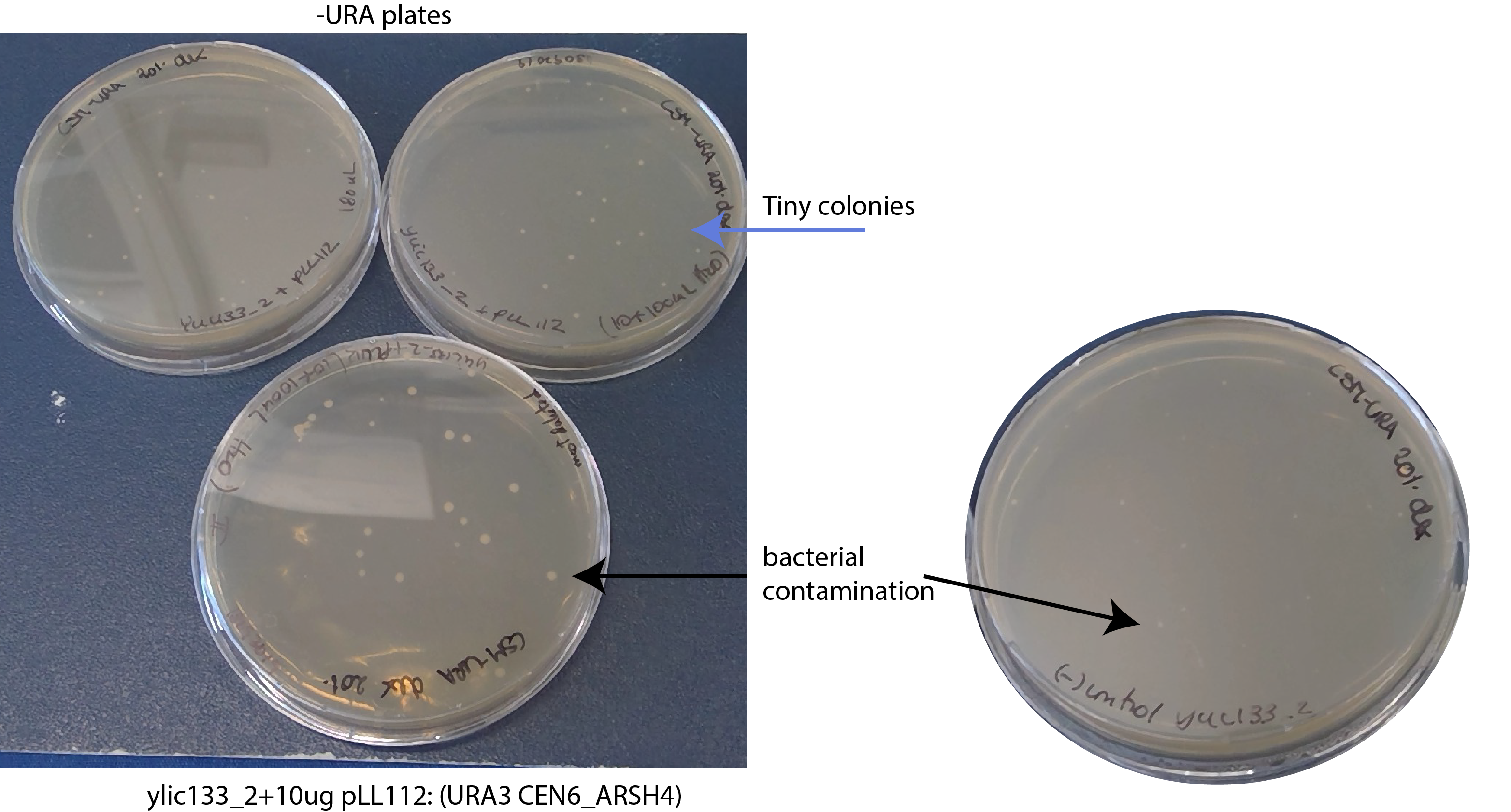
17092019- I found bacterial contamination on the plates :(
17092019- The transformants on the -ura plate are extremely tiny colonies
17092019 - Incubate ylic133-3 to redo transformation
18092019 - The liquid culture was not dense enough to do the experiment.
19092019 - I diluted the culture 1000x to do transformation next day.
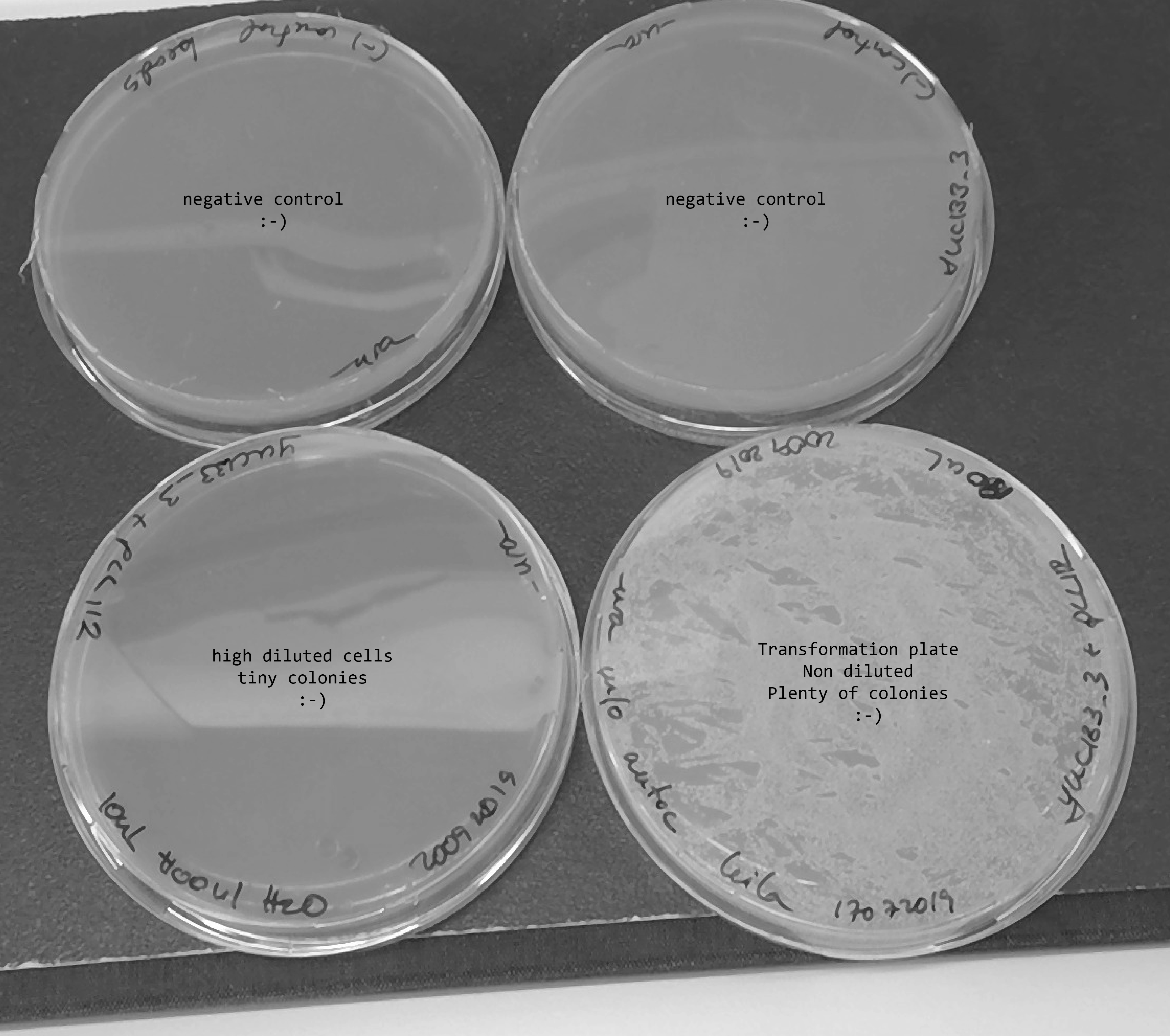
20092019 - Transformation with pLL112 (from a miniprep Ramon did the day before)
pLL112-148ng/ul , I used 10ul for transformation , hence I used 1.5 ug of plasmid
I plated two positive controls in -ura and all the negative control in -ura.
68.5. Conclusion#
I got very small transformed strains which are going to be named: ylic134=ylic133_3+pLL112 (mating type alpha, ura3+)
I inoculated transformants colonies into a CSM-URA liquid media and they did not grow , from an overnight culture. :(
It seems somehow they loose the plasmid… :pensive:
As next step I will transform ylic133 and yll3a as a positive control with pLL112, pLL55 and pLL56, to assess whether is the plasmid or the strain the bottleneck here.
