Title : Genetic checks for the Gal strains used in the Biotek
Contents
13. Title : Genetic checks for the Gal strains used in the Biotek#
13.1. Date#
30092021-
13.2. Objective#
To check for the presence of the pgal promoter in front of CDC42 for the gal strains
To check for the abscense of the endogenous CDC42 promoter in the gal strains.
To check for the abscense of BEM1 and BEM3 in the mutants .
13.3. Method#
gDNA extraction of ywkd065 a/b and ywkd071I/II
PCR
Primers :
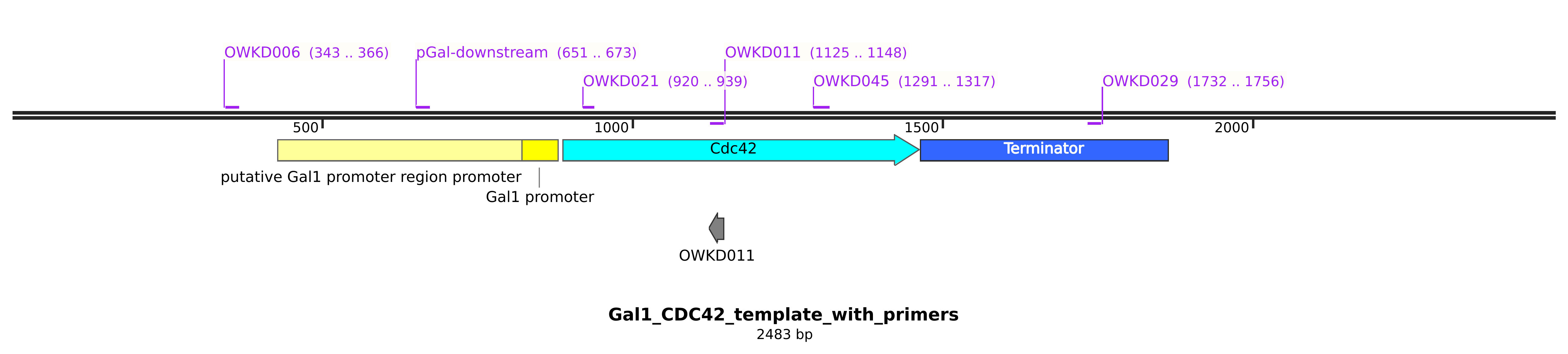
13.4. Results#
gDNA extraction
Using 3 eppies with 2mL of culture for pellet
Elution of 20ul per eppi
strain |
concentration |
|---|---|
ywkd065a |
47.6ng/ul |
ywkd065b |
51ng/ul |
ywkd071I |
40ng/ul |
ywkd071II |
40ng/ul |
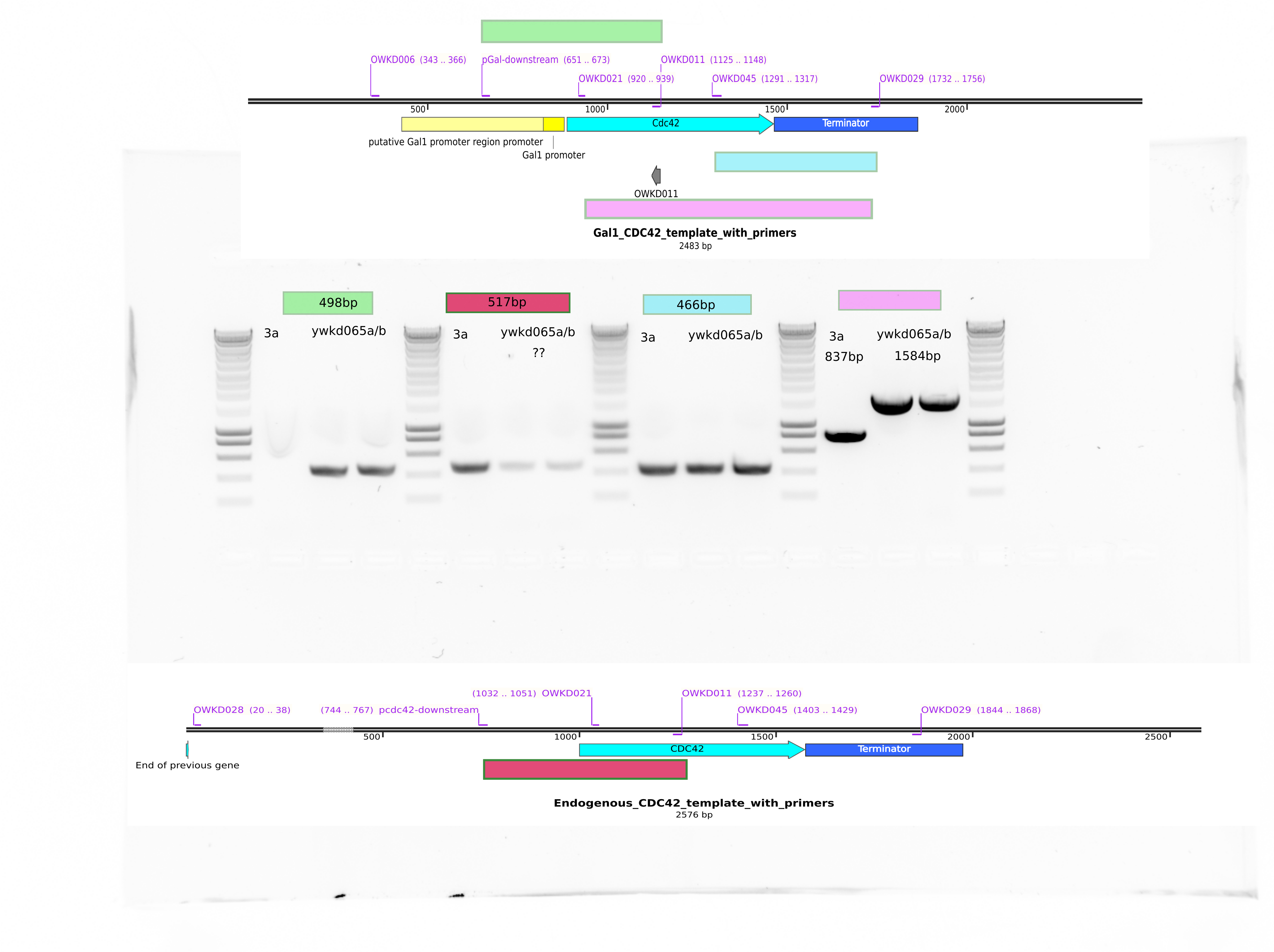
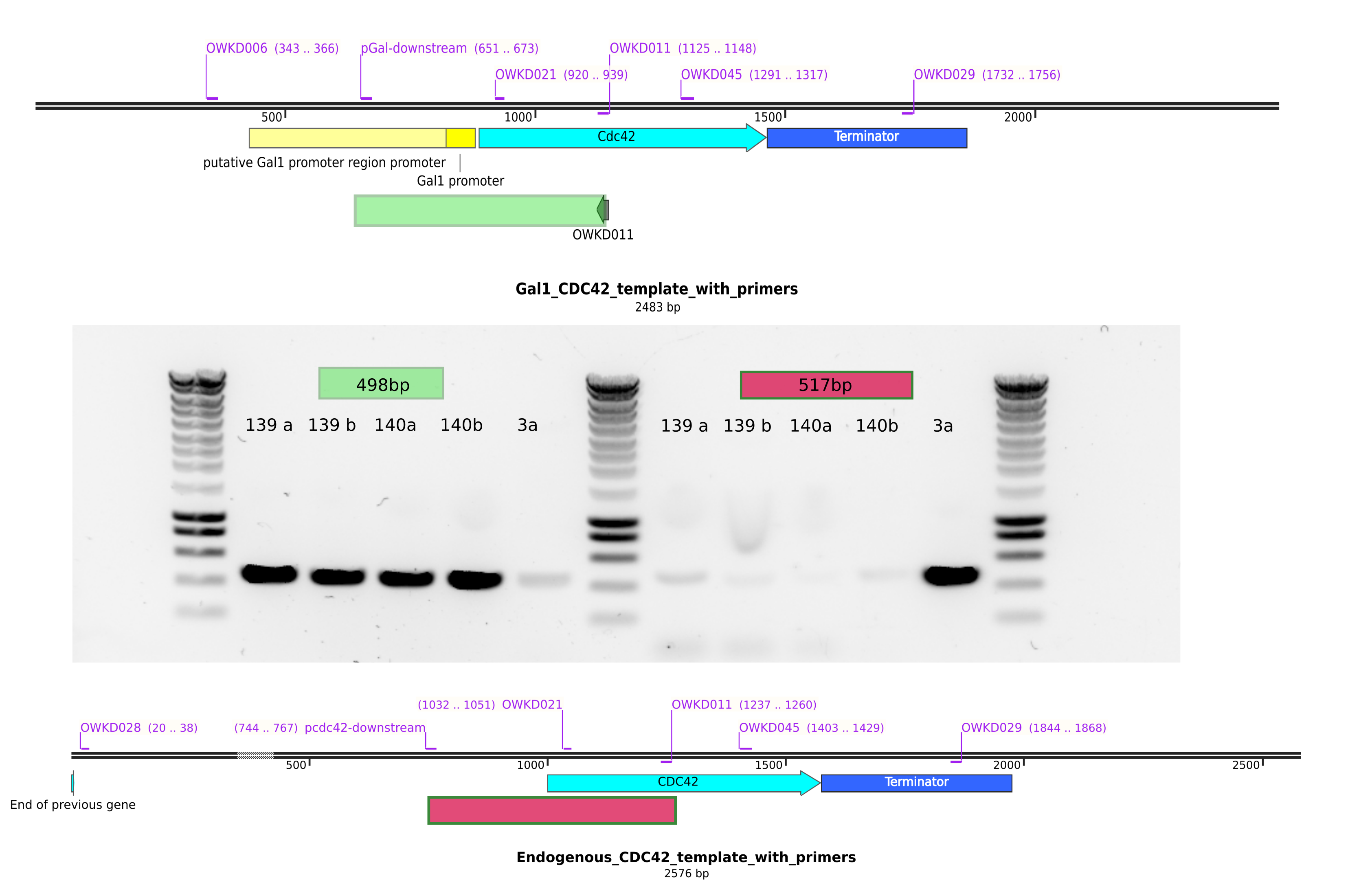
There is are suspicious bands when checking the cdc42 promoter. The primer pCdc42 downstream does not bind to the pgal construct DNA, hence I do not get the presence of this band…
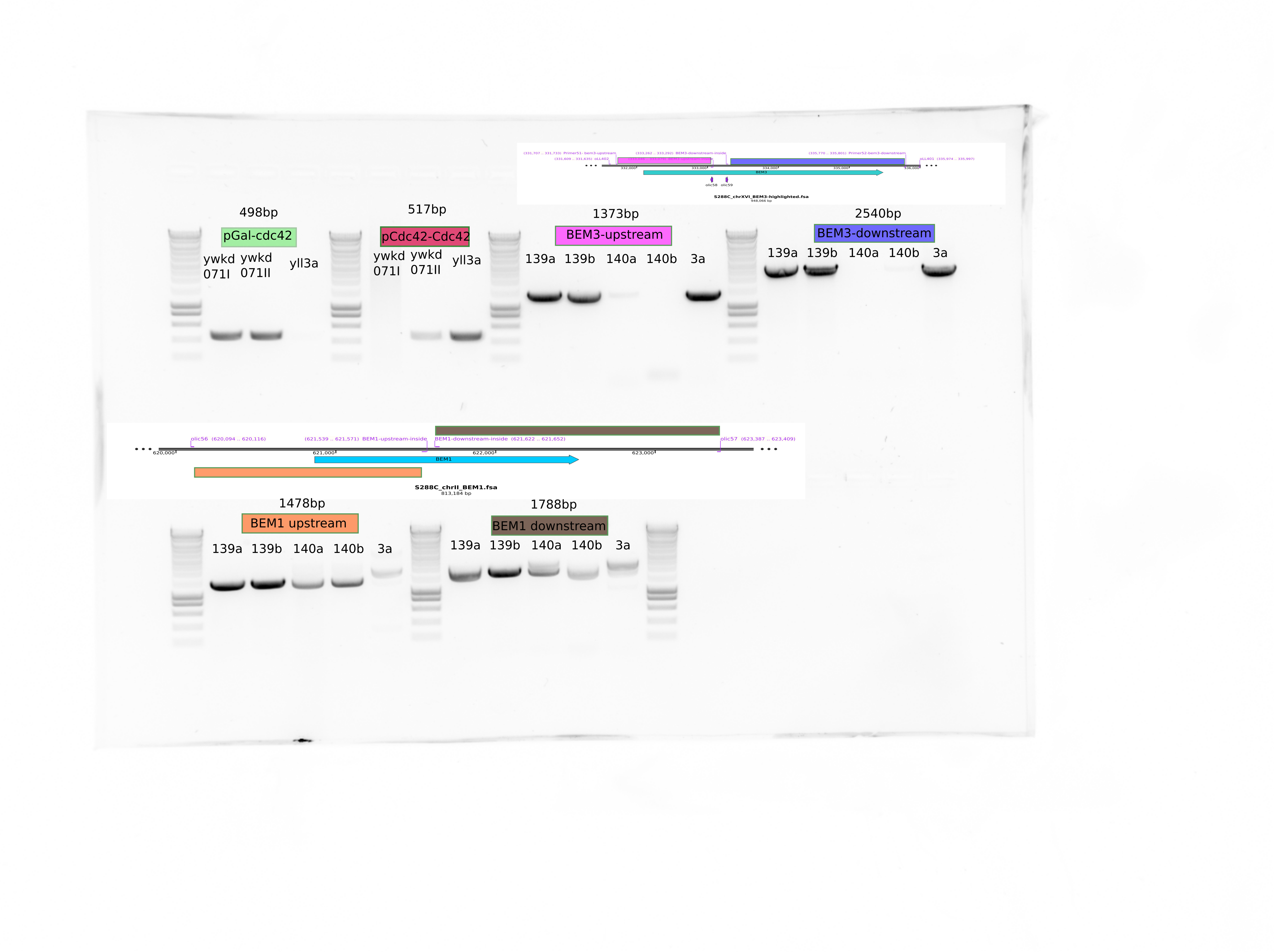
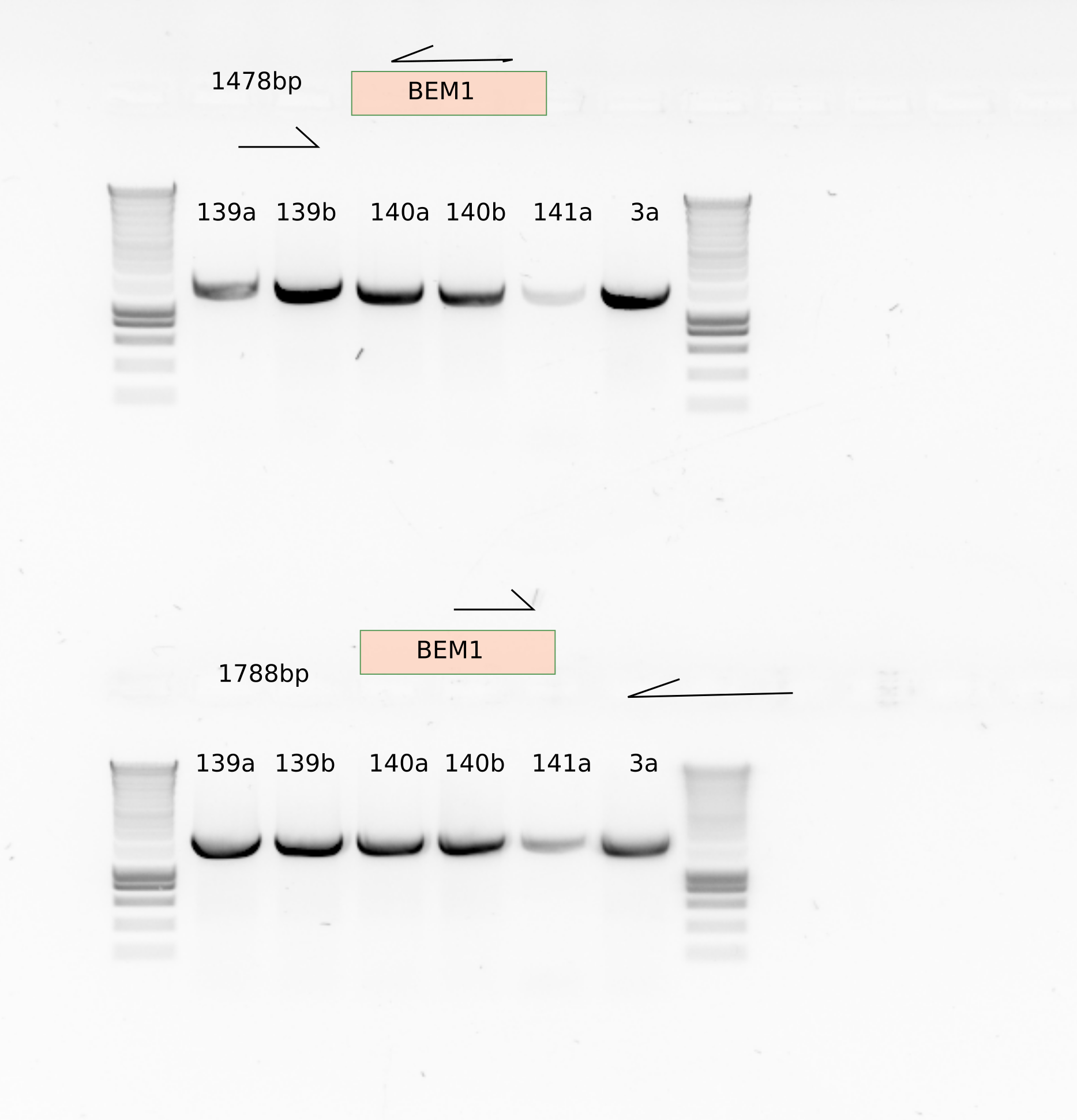
It is streaking that the mutants ylic139 and ylic140 do have BEM1!!!! (aneuploidy???)
It seems ywkd071I does not have the endogenous CDC42 promoter. So it is good.
Important note
The primers olic60/61 were wrong!!! they were binding to kanmx cassette instead of BEM1. That explains the bands in the mutants..
I ordered new primers that bind to BEM1 upstream and downstream . Let see..
13.4.1. PCR on control strains#
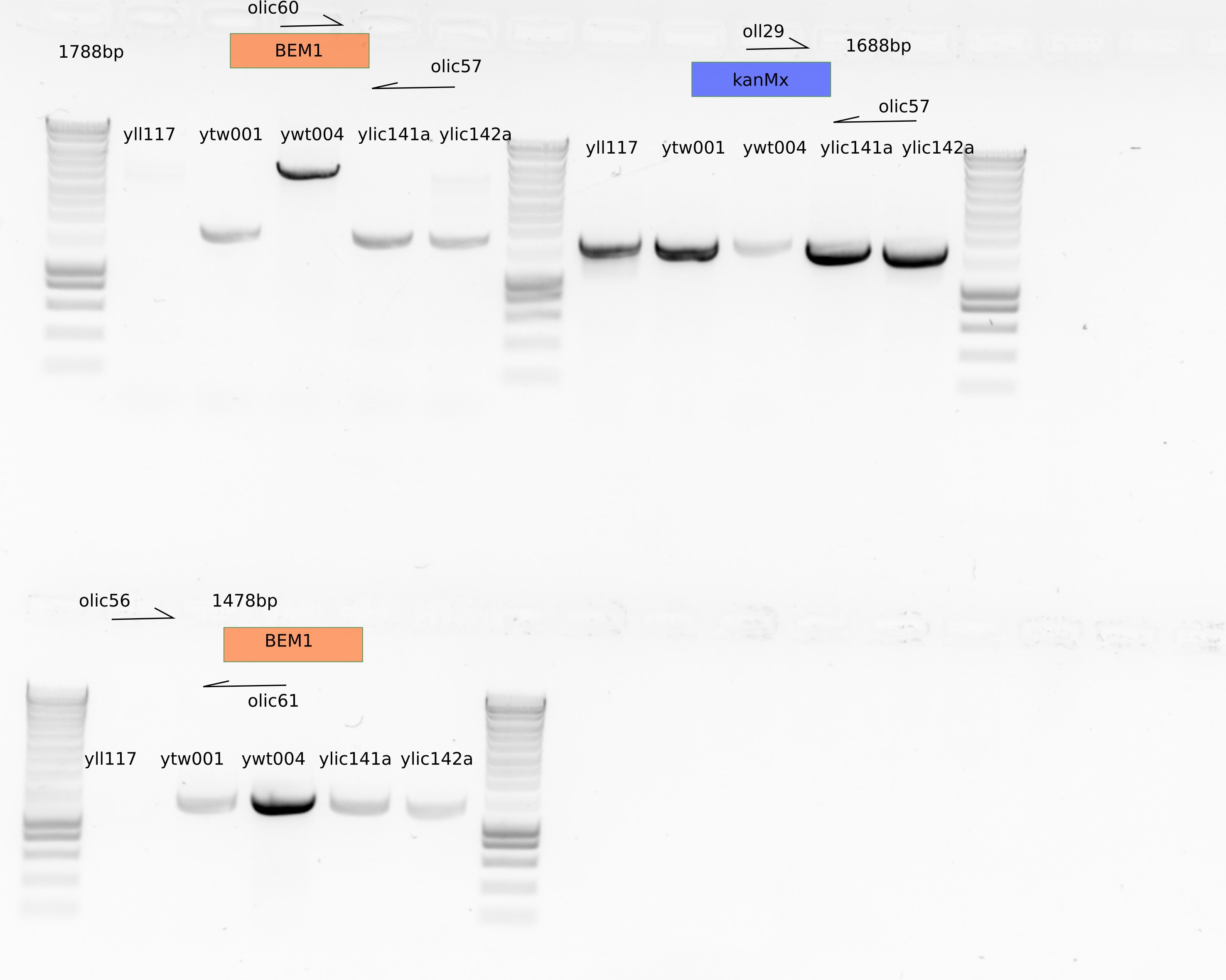
original strain used for transformation (yll117) does not show a band for BEM1. This is good.
The strain from Thomas ytw001: \(\Delta\)bem1\(\Delta\)bem3 seems to have both (I dont understand this), also my strains ylic141/ylic142 , \(\Delta\)bem1\(\Delta\)nrp1/\(\Delta\)bem1\(\Delta\)bem3\(\Delta\)nrp1
13.4.1.1. DNA extraction for ywkd017#
ywkd017:
\(\Delta\)bem1\(\Delta\)bem3\(\Delta\)nrp1
Positive control for all markers
gDNA concentration: 76ng/ul , 80ul Volume
13.5. Conclusion#
we may repeat the BEM1 transformation for :
ylic139
ylic140
ylic141
ylic142
ytw001
