Title: 03102019- III pBK549 transformation on ylic133 for further sanity check in SATAY :blush: :punch:
Contents
64. Title: 03102019- III pBK549 transformation on ylic133 for further sanity check in SATAY :blush: :punch:#
64.1. Date#
03102019-09102019
64.2. Objective#
To ensure that the constructed strain is able to pass the Satay sanity check, and then I can continue with the further steps, like mating with yEK7a.
64.3. Method#
14:00 Incubation form glycerol stocks of ylic133_1, ylic133_4,ylic133_5 and Byk832 in new YPD+6xADE media.
New pBK549 plasmid extraction from bacteria. Miniprep and enzyme restriction assay in the same day of transformation. Look here for the restriction protocol
04102019 9:15 OD measurements
OD-10X dilution |
Titer |
Dilution factor to OD=0.5 |
Time |
|
|---|---|---|---|---|
ylic133_1 |
0.368 |
3.68 |
7.76X |
9:15 |
ylic133_5 |
0.478 |
4.78 |
9.56X |
9:15 |
Byk832 |
0.057 |
0.57 |
1,14X |
9:15 |
Miniprep to extract pBK549
concentration: 46,7 ng/ul total volume 240 uL
Digestion testing by EcoRv, PvuII and both:
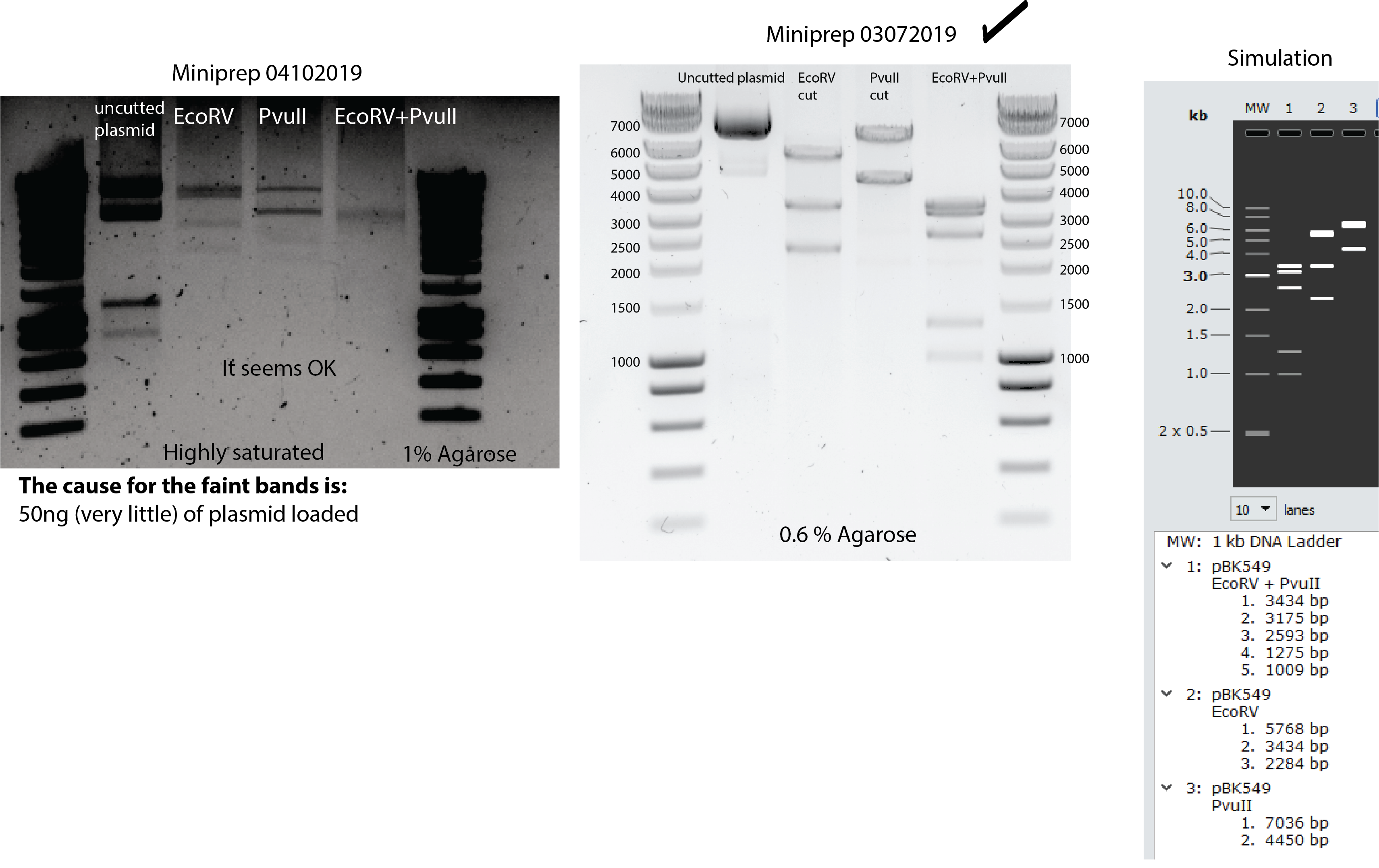
13:00 OD measurements 10x dilution
OD-10X dilution |
Titer |
Ready to transform |
Time |
|
|---|---|---|---|---|
ylic133_1 |
0.255 |
2.5 |
Yes |
9:15 |
ylic133_5 |
0.198 |
1.98 |
Yes |
9:15 |
Byk832 |
0.385 |
3.8 |
Yes |
9:15 |
3ul plasmid (46,7ng/ul) implies 140ng plasmid.
I prepare another stock of 1M of LiAc.
Plating 150ul cells+50ul MiliQ and 30ul cells+170ul MiliQ in -URA+6xADE and 20ul cells +180ul MiliQ in YPD (positive control)
64.4. Results :smile:#
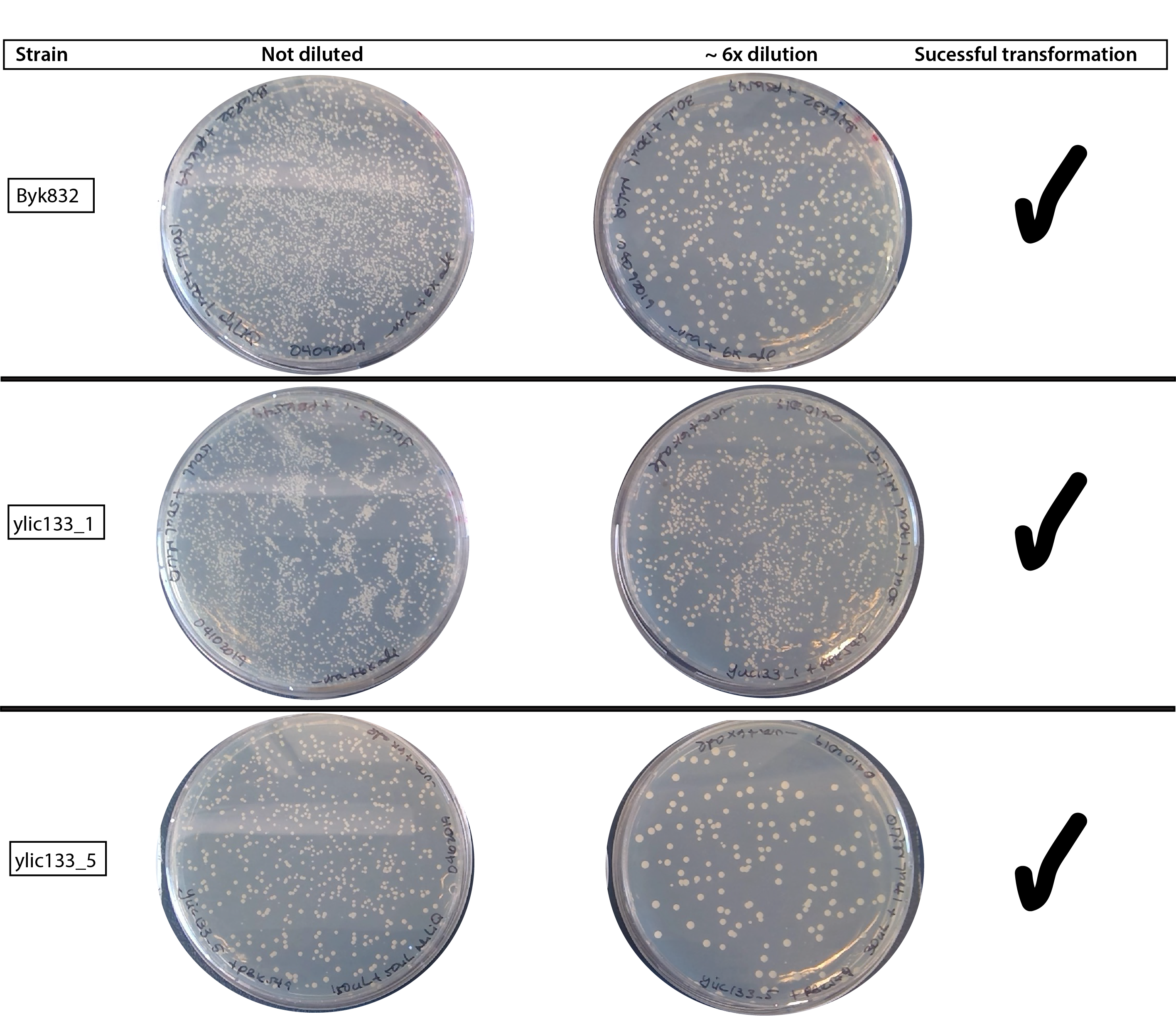
64.5. Conclusion#
09102019 This time the transformation really works , giving plenty of colonies in all the strains. I suspected that what made the big difference is the change of the LiAc.
The negative control is good, so there is no growth in any of the plates.
64.6. Next steps#
Sanity check in -ade and -ura plates .
