Title : do yeast cells show multinuclei upon CDC42 depletion? (test)
Contents
9. Title : do yeast cells show multinuclei upon CDC42 depletion? (test)#
9.1. Date#
29092022 (thursday)-
9.2. Objective#
We got a rebuttal from a reviewer asking us check the cell morphology in log phase for some galactose concentrations, to see if they look healthy.
We also got a request to check if CDC42 depletion causes multinuclei in the cells.
9.3. Method#
This implies we should do the following:
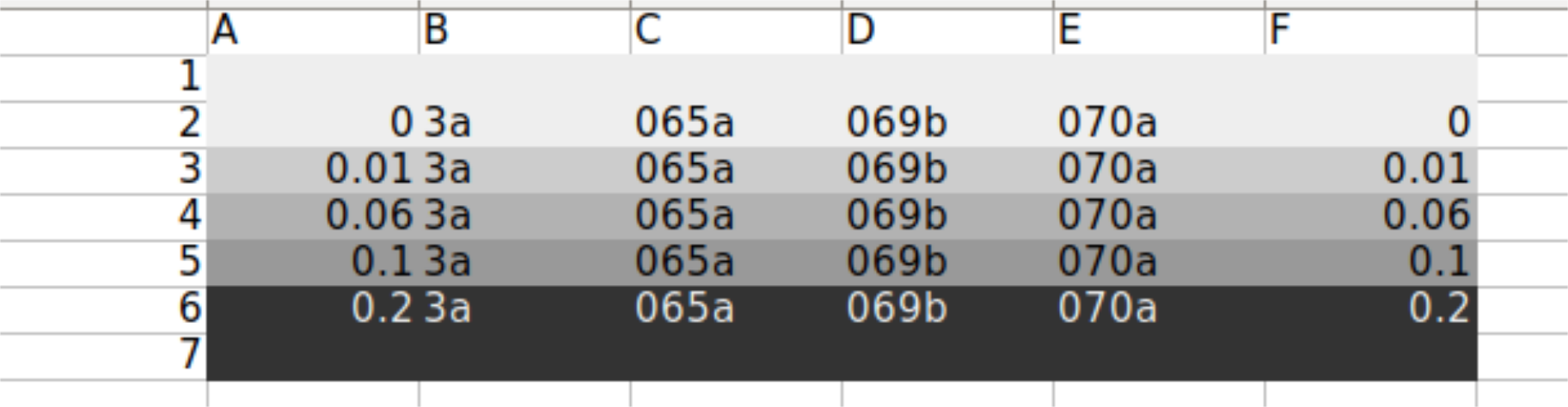
Set up a population growth experiment with the following strains: WT+sfGFP+pGal-CDC42(ywkd065a),dbem1+sfGFP+pGal-CDC42(ywkd069b),dbem1dbem3+sfGFP+pGal-CDC42(ywkd070a),WT(yll3a Control). The population growth experiment should be done considering two phases: incubation(30C)+measuring(36C).
After the measuring part , when the cells reach saturation , then we should DAPI stain them and check for multinuclei.
We will measure in : 0,0.01,0.06,0.1 amd 0.2% galactose.
For the population growth:
Incubation: 30C at 10:30am 29092022(thursday)
1ul of glycerol stock in 100ul media
Base media: 4xCSM-LF+2% Raffinose
Measuring: 36C at 10:30am 01102022(Saturday)
1ul from incubated cells in 100ul media
Base media: 4xCSM-LF+2% Raffinose
9.3.1. Plate Layout#
9.3.2. Protocol for DAPI staining#
From https://www.protocols.io/view/Yeast-DAPI-Staining-kqdg3p41l25z/v1?step=8
Grow up yeast in liquid medium overnight. The OD may not matter too much here, but something in the 0.8 - 2 range is probably ideal.
Add 333 μL (or 1 volume) yeast culture to a 1.5 mL microcentrifuge tube.
Add 666 μL (or 2 volume) of 100% Ethanol to the 1.5 mL microcentrifuge tube.
Let the yeast ethanol mixture sit at room temperature for 30-60 minutes.
Spin down yeast cells for 1 minute at 2500 RPM.
Pour out the supernatant and resuspend the pellet in 1mL of 1 x PBS(1X PBS (Phosphate-buffered saline )), then centrifuge for 1 minute at 2500 RPM.
Pour out the supernatant and resuspend the pellet in 200 μL of a 1 x PBS / 1:2000 Dilution DAPI mixture 1.
Add one drop of the yeast suspended in the PBS / DAPI mixture onto a microscope slide, add a coverslip on top, and go observe the stained yeast. Make sure after you do this part to go look at it within a few hours of resuspending in PBS / DAPI. The sooner you can get to a microscope the better.
9.4. Results#
9.5. Conclusion#
- 1
Add 1 mL 1 x PBS to a 1.5 mL microcentrifuge tube.Add 0.5 μL of a 2.5 mg/mL (or a 1:2000 dilution) of DAPI to the 1 x PBS.
